

CHROMOCELL THERAPEUTICS CORPORATION 1 CHROMOCELL THERAPEUTICS CORPORATION Potential Breakthrough Drug for Erythromelalgia and Other Pain Indications Issuer Free Writing Prospectus dated, November 7 , 2023 Filed Pursuant to Rule 433 of the Securities Act 1933 , as amended Relating to Preliminary Prospectus dated November 7 , 2023 Registration Statement No . 333 - 2691880
CHROMOCELL THERAPEUTICS CORPORATION 2 Legal Disclaimer This presentation of Chromocell Therapeutics Corporation (“we”, “us”, “our” or the “Company”) contains “forward - looking statements” within the meaning of the Private Securities Litigation Reform Act and other securities laws . Words such as “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict, “project,” “seek,” “should,” “target,” “will,” “would” or similar expressions and the negatives of those term are intended to identify forward - looking statements . Forward - looking statements reflect management’s current expectations, are based on judgments and assumptions, are inherently uncertain and are subject to risks, uncertainties and other factors, which could cause the Company’s actual results, performance or achievements to differ materially from expected future results, performance or achievements expressed or implied in those forward - looking statements . Examples of these forward - looking statements and the related risks, uncertainties and other factors include, but are not limited to, the following : the timing, progress and results of preclinical and clinical trials for CC 8464 , its estimates regarding the potential market opportunity for CC 8464 , its ability to develop CC 8464 and future compounds, its ability to protect its intellectual property and enforce its intellectual property rights, and its ability to execute its development strategy and sustain its competitive position . Actual future results and trends may differ materially depending on a variety of factors, including, but not limited to : the Company’s limited operating history, the Company’s ability to develop CC 8464 , the Company’s ability to establish its market development capabilities to commercialize its products and generate any revenue, and the Company’s ability to obtain regulatory approval of CC 8464 . Forward - looking statements are provided to allow potential investors the opportunity to understand management’s beliefs and opinions in respect of the future so that they may use such beliefs and opinions as one factor in evaluating an investment . These statements are not guarantees of future performance and undue reliance should not be placed on them . For a more detailed description of the risks and uncertainties affecting the Company, please review the Company’s Registration Statement on Form S - 1 , as amended (File No . 333 - 269188 ) and other documents that may be filed from time to time with the SEC, including, but not limited to, the risks detailed in the Company’s preliminary prospectus dated November 7 , 2023 , as a part of our Registration Statement . Any forward - looking statement in this presentation, in any related presentation supplement and in any related free writing presentation reflects our current view with respect to future events and is subject to these and other risks, uncertainties and assumptions relating to our business, results of operations, industry and future growth . You should read this presentation with the understanding that our actual future results may be materially different from any future results expressed or implied by these forward - looking statements . Except as required by law, we assume no obligation to update or revise these forward - looking statements for any reason, even if new information becomes available in the future . The issuer has filed a registration statement (including a prospectus) with the SEC for the offering to which this communication relates . Before you invest, you should read the prospectus in that registration statement and other documents the issuer has filed with the SEC for more complete information about the issuer and this offering . A copy of the preliminary prospectus can be found for free by visiting EDGAR on the SEC website at www . sec . gov . Alternatively, the issuer, any underwriter or any dealer participating in the offering will arrange to send you the prospectus if you request it by calling Titan Partners Group LLC, a division of American Capital Partners, LLC at ( 929 ) 833 - 1246 or info@titanpartnersgrp . com . This presentation provides basic information about the Company and the offering . Because it is only a summary, this presentation does not cover all the information that should be considered before investing . You should read carefully the factors described in the “Risk Factors : section of the prospectus contained in the Company’s Registration Statement to better understand the risks and uncertainties inherent in our business and any forward - looking statements .
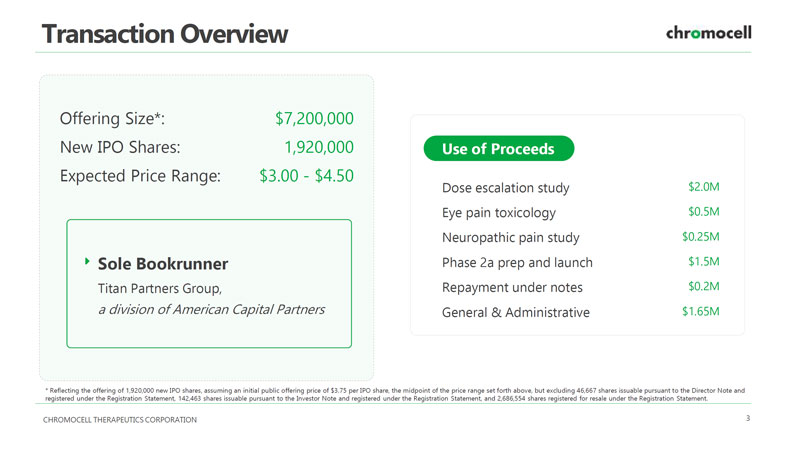
CHROMOCELL THERAPEUTICS CORPORATION 3 Transaction Overview Offering Size*: $7,200,000 New IPO Shares: 1,920,000 Expected Price Range: $3.00 - $4.50 Sole Bookrunner Titan Partners Group, a division of American Capital Partners Dose escalation study $2.0M Eye pain toxicology $0.5M Neuropathic pain study $0.25M Phase 2a prep and launch $1.5M Repayment under notes $0.2M Use of Proceeds General & Administrative $1.65M * Reflecting the offering of 1 , 920 , 000 new IPO shares, assuming an initial public offering price of $ 3 . 75 per IPO share, the midpoint of the price range set forth above, but excluding 46 , 667 shares issuable pursuant to the Director Note and registered under the Registration Statement, 142 , 463 shares issuable pursuant to the Investor Note and registered under the Registration Statement, and 2 , 686 , 554 shares registered for resale under the Registration Statement .
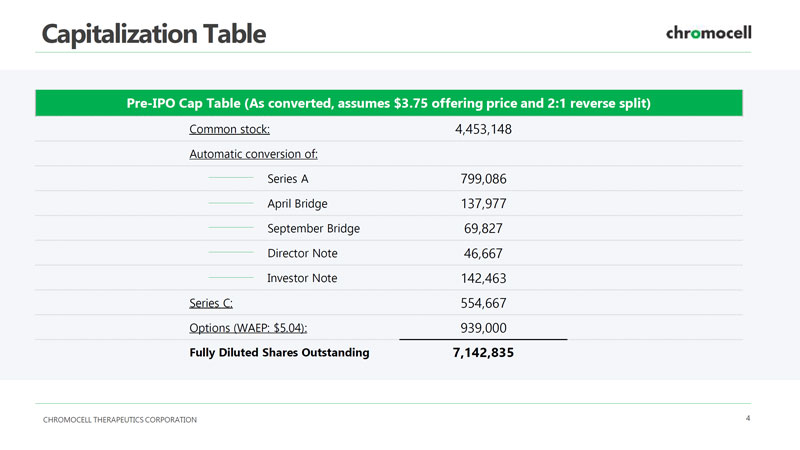
CHROMOCELL THERAPEUTICS CORPORATION 4 Capitalization Table Pre - IPO Cap Table (As converted, assumes $3.75 offering price and 2:1 reverse split) Common stock: 4,453,148 Automatic conversion of: Series A 799,086 April Bridge 137,977 September Bridge 69,827 Director Note 46,667 Investor Note 142,463 Series C: 554,667 Options (WAEP: $5.04): 939,000 Fully Diluted Shares Outstanding 7,142,835

CHROMOCELL THERAPEUTICS CORPORATION 5 Investment Highlights Overview Phase II life sciences company focused on developing patented non - opioid pain treatment compound (CC8464) through sodium channel blockade (NAV) Strategy Advance CC8464 towards commercialization, initially focusing on orphan designated Erythromelalgia (“EM”) and eye pain and plans to pursue neuropathic pain Unmet Needs • EM is a rare / orphan disease population with no current effective clinical treatments • Eye pain is larger market with significant unmet medical need Clinical • Phase 1 completed, fast track designation granted • 3 months toxicology completed; 3 Phase I studies completed with 165 patients • Run clinical trials on a tax advantaged / cost basis Catalysts Use of proceeds for the offering to fund the dose escalation study, in vivo studies for treatment of eye pain and to ramp up a Phase II proof - of - concept study for CC8464.
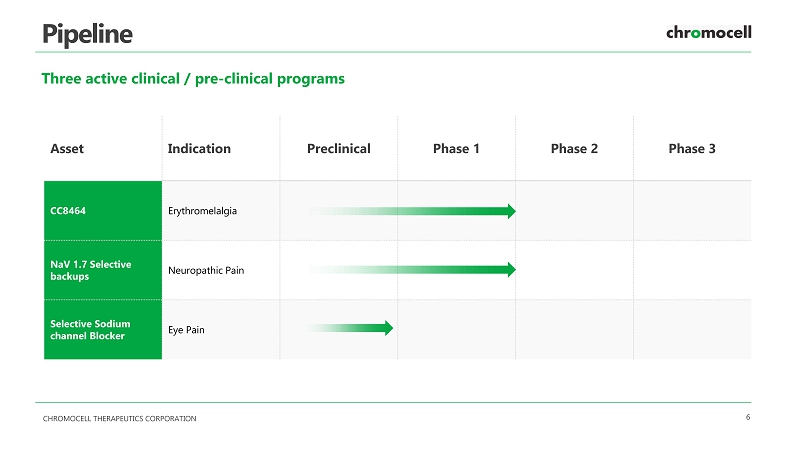
CHROMOCELL THERAPEUTICS CORPORATION 6 Pipeline Three active clinical / pre - clinical programs Asset Indication Preclinical Phase 1 Phase 2 Phase 3 Erythromelalgia Neuropathic Pain Eye Pain

CHROMOCELL THERAPEUTICS CORPORATION 7 Management Team Frank Knuettel | Interim CEO & Chief Financial Officer Mr . Knuettel has 30 years of management experience in growing early - stage companies . He has raised more than $ 300 million via venture, public equity and debt offerings and managed more than 15 mergers and acquisition transactions along with large - scale licensing transactions with fortune 50 companies . Mr . Knuettel holds numerous board positions, at both public and private companies, including 180 Life Sciences (ATNF), ECOM Medical, Murphy Canyon Acquisition Corp . (MURF) and Relativity Acquisition Corp . (RACY) . He holds an MBA from The Wharton School and a BA from Tufts University . Dr. Eric Lang | Chief Medical Officer Dr . Lang is an Anesthesiologist and Pain Management Specialist with over 26 years of experience in the pharmaceutical industry . During his pharmaceutical career, he has had both broad - based drug and device development expertise in a variety of therapeutic areas . Dr . Lang has experience in designing development programs from early translational stages through phase III including the successful filing of several recent INDs and NDAs . Dr . Lang began his career with J&J and later worked for Novartis, Javelin Pharmaceuticals, Grunenthal USA, Covance, EnteraBio and Nevakar Inc . Dr . Lang earned his MD from Ben Gurion University, Israel and completed post graduate training at Emory University in Atlanta . Christian Kopfli | Vice Chairman and Chief Strategy Officer Mr . Kopfli co - founded Chromocell Corporation (former parent company) in 2002 , becoming Chief Executive Officer in 2005 . Prior to joining Chromocell , he was an Associate at Davis Polk & Wardwell, working in its New York City, Tokyo and Frankfurt offices . At Davis Polk, Christian worked extensively in M&A, Capital Markets and Private Equity Transactions . He received a doctoral degree in law (magna cum laude) from the University of Zurich in 2000 and earned his LL . M . from Columbia Law School in New York City in 1998 . Christian is a Board Member of BioNJ and admitted to the Bar in New York and Switzerland .

CHROMOCELL THERAPEUTICS CORPORATION 8 CHROMOCELL THERAPEUTICS CORPORATION Development Plan - Erythromelalgia
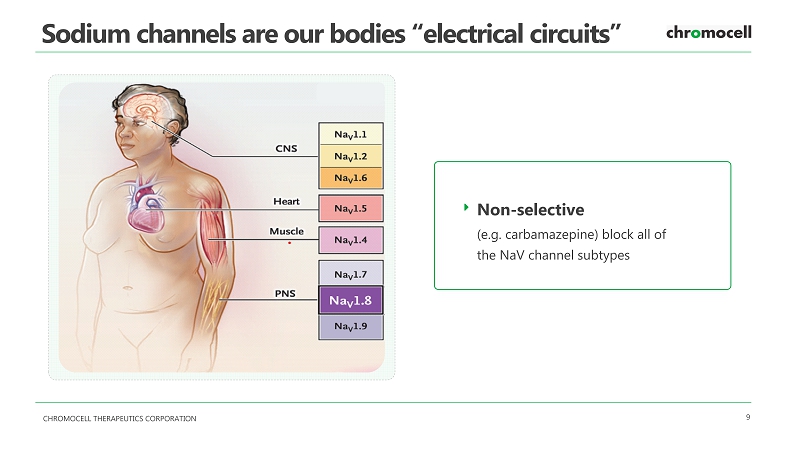
CHROMOCELL THERAPEUTICS CORPORATION 9 Sodium channels are our bodies “electrical circuits” Non - selective (e.g. carbamazepine) block all of the NaV channel subtypes
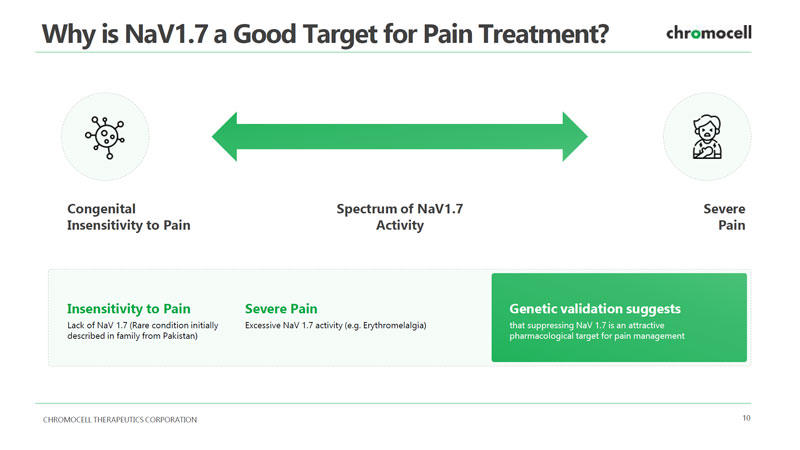
CHROMOCELL THERAPEUTICS CORPORATION 10 Why is NaV1.7 a Good Target for Pain Treatment? Insensitivity to Pain Lack of NaV 1.7 (Rare condition initially described in family from Pakistan) Severe Pain Excessive NaV 1.7 activity (e.g. Erythromelalgia) Genetic validation suggests that suppressing NaV 1.7 is an attractive pharmacological target for pain management Congenital Insensitivity to Pain Spectrum of NaV1.7 Activity Severe Pain

CHROMOCELL THERAPEUTICS CORPORATION 11 CC8464 – Development Status Preclinical • Potent ( nM ) inhibitor of human NaV1.7; Subtype selective • Demonstrated in vivo efficacy in several rodent models of pain: Acute, chronic neuropathic, inflammatory, visceral and post - surg ical • No CNS and muscle/motor dysfunction effects CMC • Drug Substance: scaled up, cGMP API available • Drug Product: tablet (active, 3 strengths and placebo) available for Phase 2. Potency verification to be confirmed. Tox • Did not exhibit genotoxicity • Tox data supports up to 3 - month dosing in human clinical trials Clinical • Phase 1 completed • Occurrence of rashes may be addressed with gradual dose - escalation protocols • Clinical data supports a Proof of Concept (“POC”) EM study • Seek orphan drug designation and apply for breakthrough status

CHROMOCELL THERAPEUTICS CORPORATION 12 Erythromelalgia Clinical – Overview Symptoms Erythema (heat), Pain (Usually severe burning pain but may include pins and needles or itching), Swelling, Change in perspira tio n and discoloration Types of EM Primary EM (Inherited or Sporadic SCN9A mutations + non genetic or uncharacterized) or Secondary EM (Related to an underlying disease, toxin or drug induced) Treatment No known currently approved treatments and off - label treatments often ineffective Prevalence Estimated between 4,000 – 50,000 EM patients in the US with patents covering approximately 4.2 billion people worldwide Neurovascular condition affecting the feet, hands, face, or other parts of the body triggered by warmth, physical activity or stress Intolerance to exercise, warm baths/showers and clothing In severe cases, the disease may lead to depression, anxiety and suicidal tendencies .
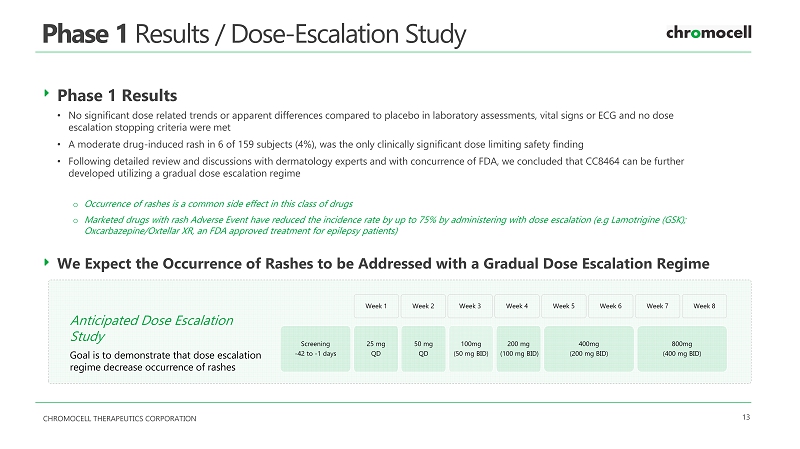
CHROMOCELL THERAPEUTICS CORPORATION 13 Phase 1 Results / Dose - Escalation Study Phase 1 Results • No significant dose related trends or apparent differences compared to placebo in laboratory assessments, vital signs or ECG and no dose escalation stopping criteria were met • A moderate drug - induced rash in 6 of 159 subjects (4%), was the only clinically significant dose limiting safety finding • Following detailed review and discussions with dermatology experts and with concurrence of FDA, we concluded that CC8464 can be further developed utilizing a gradual dose escalation regime o Occurrence of rashes is a common side effect in this class of drugs o Marketed drugs with rash Adverse Event have reduced the incidence rate by up to 75% by administering with dose escalation ( e.g Lamotrigine (GSK); Oxcarbazepine/ Oxtellar XR, an FDA approved treatment for epilepsy patients) Anticipated Dose Escalation Study Goal is to demonstrate that dose escalation regime decrease occurrence of rashes Screening - 42 to - 1 days 25 mg QD 50 mg QD 100mg (50 mg BID) 200 mg (100 mg BID) 400mg (200 mg BID) 800mg (400 mg BID) Week 1 Week 2 Week 3 Week 8 Week 7 Week 6 Week 4 Week 5 We Expect the Occurrence of Rashes to be Addressed with a Gradual Dose Escalation Regime
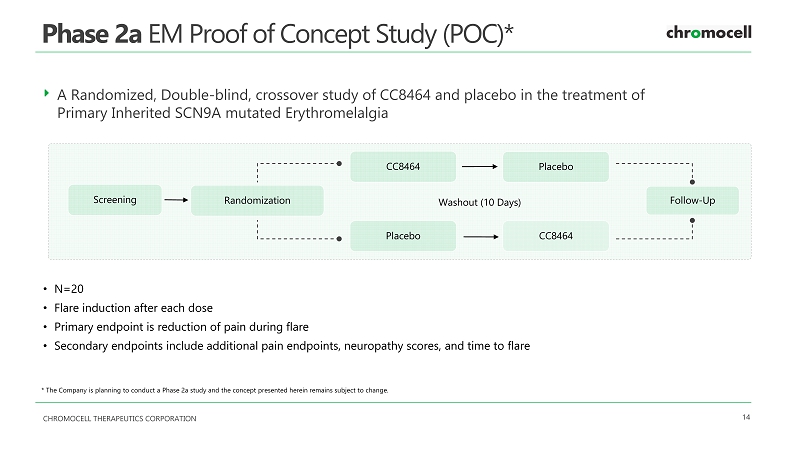
CHROMOCELL THERAPEUTICS CORPORATION 14 Phase 2a EM Proof of Concept Study (POC)* * The Company is planning to conduct a Phase 2 a study and the concept presented herein remains subject to change . A Randomized, Double - blind, crossover study of CC8464 and placebo in the treatment of Primary Inherited SCN9A mutated Erythromelalgia Screening Randomization CC8464 CC8464 Placebo Placebo Washout (10 Days) Follow - Up • N= 20 • Flare induction after each dose • Primary endpoint is reduction of pain during flare • Secondary endpoints include additional pain endpoints, neuropathy scores, and time to flare
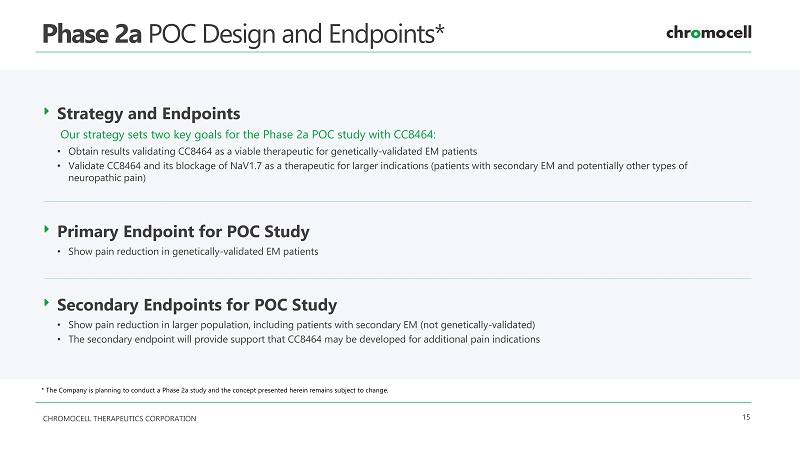
CHROMOCELL THERAPEUTICS CORPORATION 15 Phase 2a POC Design and Endpoints* Strategy and Endpoints Our strategy sets two key goals for the Phase 2a POC study with CC8464: • Obtain results validating CC8464 as a viable therapeutic for genetically - validated EM patients • Validate CC8464 and its blockage of NaV1.7 as a therapeutic for larger indications (patients with secondary EM and potentiall y o ther types of neuropathic pain) Primary Endpoint for POC Study • Show pain reduction in genetically - validated EM patients Secondary Endpoints for POC Study • Show pain reduction in larger population, including patients with secondary EM (not genetically - validated) • The secondary endpoint will provide support that CC8464 may be developed for additional pain indications * The Company is planning to conduct a Phase 2 a study and the concept presented herein remains subject to change .
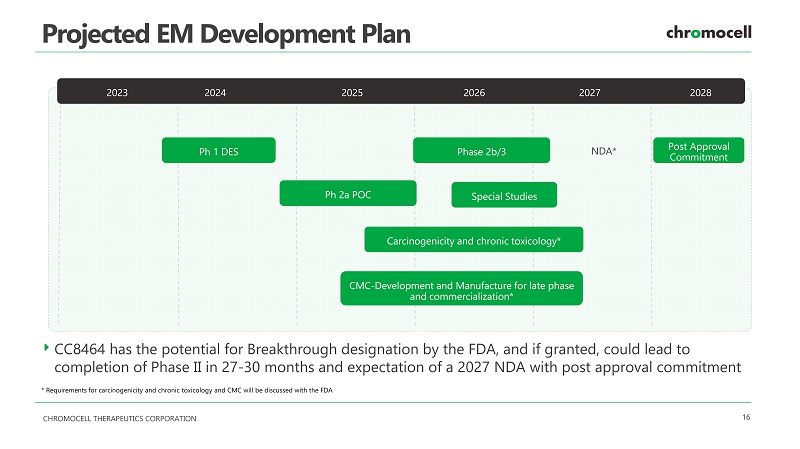
CHROMOCELL THERAPEUTICS CORPORATION 16 Projected EM Development Plan CC8464 has the potential for Breakthrough designation by the FDA, and if granted, could lead to completion of Phase II in 27 - 30 months and expectation of a 2027 NDA with post approval commitment NDA* Ph 1 DES Phase 2b/3 Post Approval Commitment 2023 2024 2025 2026 2027 2028 Ph 2a POC Special Studies Carcinogenicity and chronic toxicology* CMC - Development and Manufacture for late phase and commercialization* * Requirements for carcinogenicity and chronic toxicology and CMC will be discussed with the FDA
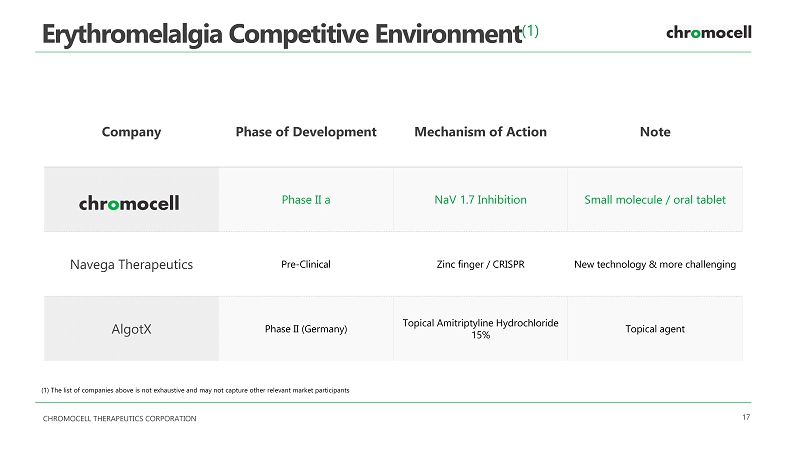
CHROMOCELL THERAPEUTICS CORPORATION 17 Erythromelalgia Competitive Environment (1) Company Phase of Development Mechanism of Action Note Phase II a NaV 1.7 Inhibition Small molecule / oral tablet Navega Therapeutics Pre - Clinical Zinc finger / CRISPR New technology & more challenging AlgotX Phase II (Germany) Topical Amitriptyline Hydrochloride 15% Topical agent ( 1 ) The list of companies above is not exhaustive and may not capture other relevant market participants

CHROMOCELL THERAPEUTICS CORPORATION 18 CHROMOCELL THERAPEUTICS CORPORATION CC8464: Potential Additional Indications and Development Budget
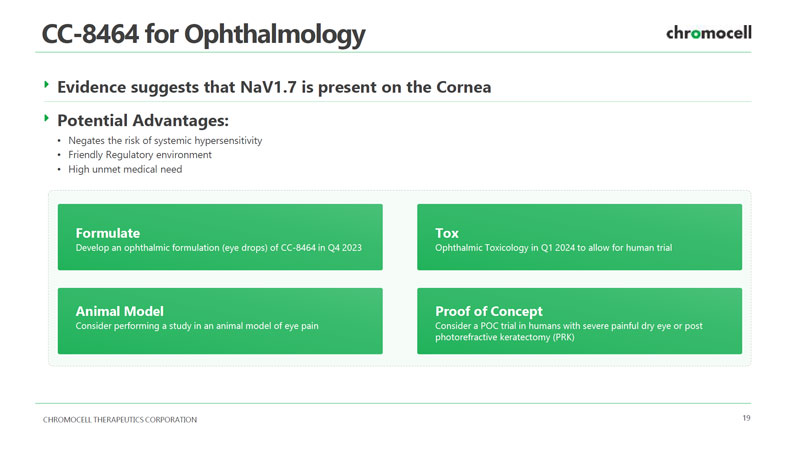
CHROMOCELL THERAPEUTICS CORPORATION 19 CC - 8464 for Ophthalmology Formulate Develop an ophthalmic formulation (eye drops) of CC - 8464 in Q4 2023 Potential Advantages: • Negates the risk of systemic hypersensitivity • Friendly Regulatory environment • High unmet medical need Evidence suggests that NaV1.7 is present on the Cornea Animal Model Consider performing a study in an animal model of eye pain Tox Ophthalmic Toxicology in Q1 2024 to allow for human trial Proof of Concept Consider a POC trial in humans with severe painful dry eye or post photorefractive keratectomy (PRK)

CHROMOCELL THERAPEUTICS CORPORATION 20 CC - 8464 for Neuropathic Pain Animal Models Animal models of neuropathic pain conducted by Chromocell suggest that CC - 8464 is likely to be effective in several types of Neuropathic Pain NaV1.7 has good potential for the treatment of Neuropathic Pain Tox Existing toxicology studies conducted by Chromocell are sufficient to support initiation of proof - of - concept trial in Neuropathic Pain Proof of Concept Developing a proof - of - concept trial in humans with neuropathic pain

CHROMOCELL THERAPEUTICS CORPORATION 21 CHROMOCELL THERAPEUTICS CORPORATION Board of Directors & Scientific Advisory Board

CHROMOCELL THERAPEUTICS CORPORATION 22 Independent Board of Directors Todd Davis | Chairman Mr . Davis is Chief Executive Officer and a member of the Board of Directors of Ligand Pharmaceuticals and has nearly 30 years of experience in biopharmaceutical and life sciences operations and investing . He has been involved in over $ 3 billion of healthcare financings including growth equity, public equity turnarounds, structured debt and royalty acquisitions . He has led, structured and closed more than 40 intellectual property licenses, as well as royalty and hybrid royalty - debt transactions . Mr . Davis is a navy veteran and holds a B . S . from the U . S . Naval Academy and an M . B . A . from Harvard University . Ezra Friedberg Mr . Friedberg has served as a member of our Board since May 2021 . Ezra is a seasoned investor with more than twenty years of investing experience in both public and private companies . He invests actively in the biotech space and has served on the board of directors of Humanigen (HGEN), a clinical - stage biopharmaceutical company which develops monoclonal antibodies . Mr . Friedberg is a graduate of Johns Hopkins University . Dr. Richard Malamut Dr . Malamut is currently CMO at MedinCell Inc . He has extensive experience focusing on early clinical development in Neurology, Psychiatry and Analgesia at Collegium Pharmaceuticals, Braeburn Pharmaceuticals, Teva, Bristol - Myers Squibb and AstraZeneca . Dr . Malamut earned his medical degree from Hahnemann University and completed both a residency in Neurology and a fellowship in Neuromuscular disease . He worked as a board - certified neurologist and has more than 50 publications in the fields of pain medicine, neuromuscular disease, autonomic disease, and neurodegenerative disease . Chia - Lin Simmons Ms . Simmons is the CEO of LogicMark , Inc . (Nasdaq : LGMK), the former CEO at LookyLoo and a former executive at Google, Harman International and Amazon . She is a current Board Member of New Energy Nexus, an international NGO that support clean energy entrepreneurs . Ms . Simmons graduated Magna cum Laude and Phi Beta Kappa from U . C . San Diego . She received her MBA from Cornell University, where she was a Park Leadership Fellow and her JD from George Mason University School of Law .
CHROMOCELL THERAPEUTICS CORPORATION 23 Scientific Advisory Board (“SAB”) Stephen G. Waxman | MD, PhD, Yale School of Medicine, Chairman • Bridget Marie Flaherty Professor of Neurology, Neuroscience, and Pharmacology • Chair, Department of Neurology ( 1986 - 2009 ), Yale University School of Medicine • Director, Center for Neuroscience & Regeneration Research, Yale Robert H. Dworkin | PhD, University of Rochester Medical Center • Adjunct Senior Scientist, Department of Anesthesiology, Critical Care & Pain Management, Hospital for Special Surgery Research Institute, New York, NY • Director, Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks, and Pediatric Anesthesia Safety Initiative public - private partnership with the FDA • Editorial Boards : Canadian Journal of Pain, Journal of Pain